
Next year marks the final year of the United Nations Millennium Development Goals initiative. As we near this milestone, there is some cause for celebration. Thanks to the goal galvanising efforts to defeat malaria, deaths from this mosquito-borne disease have dropped by 42 per cent since 2000. Fighting malaria has made a positive impact on other goals too, including that to reduce child deaths.
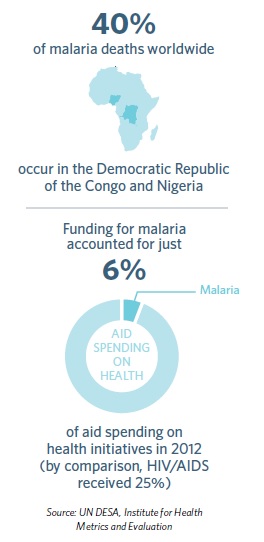
With the funding, resources and scale of the global heath community trained on malaria, increasing use of bed nets, anti-malarial treatments and insecticide sprays have all helped to push the disease back. Reducing cases can help ease the economic burden of malaria – particularly in sub-Saharan Africa, where up to 40 per cent of healthcare spending goes on fighting the disease.
Despite this progress, there is no room for complacency. Malaria is insidious. It still claims more than 600,000 lives each year, mostly those of young children in Africa, where every minute, a child dies from malaria. Rising resistance to treatments and insecticides and potential funding cuts mean that any foothold against the disease is fragile.
 If we are to defeat malaria for good, new interventions that complement our existing methods – such as bed nets and medicines – are needed. To control malaria, we need more ammunition, and that could come in the form of a vaccine.
If we are to defeat malaria for good, new interventions that complement our existing methods – such as bed nets and medicines – are needed. To control malaria, we need more ammunition, and that could come in the form of a vaccine.
Currently, no vaccine exists against malaria – or any human parasite. Finding ways to overcome the malaria parasite’s defence mechanisms is extraordinarily challenging. But for the last 30 years, GlaxoSmithKline (GSK) has been striving to unlock these secrets and develop a vaccine. Now we are closer than ever to fulfilling that goal.
In July this year, we filed a regulatory application for our malaria vaccine candidate – RTS,S – to the European Medicines Agency (EMA). This follows a large-scale, late-stage study of RTS,S in over 15,000 children across seven countries in Africa. Results showed that after 18 months of follow-up – on top of the use of bed nets – RTS,S almost halved the number of malaria cases in young children aged 5–17 months (at first vaccination) and reduced cases by almost a quarter in babies aged 6–12 weeks (at first vaccination).
Although the vaccine candidate has only partial efficacy, given the huge public health burden and economic cost of malaria in Africa, it could make a significant impact on the health and wealth of the continent.
Submitting a file to the EMA marks the first step in the regulatory process towards making RTS,S available as an additional tool to help prevent malaria in African children. The agency will now thoroughly review the evidence collected, in collaboration with the World Health Organization (WHO).
If, after its scientific evaluation, the EMA gives a positive opinion, WHO indicated it would consider introducing a policy recommendation for RTS,S by the end of 2015. This would pave the way for national regulatory applications in Africa and for African countries to embed the vaccine in their immunisation programmes, if they decide to do so.
As well as the scientific challenge of developing a vaccine, there are economic challenges too. Ultimately, RTS,S would be used by young children in Africa – who could never be expected to pay for it. That is why, should RTS,S be recommended, African countries and international organisations should be able to purchase the vaccine at a level so that it can be free at the point of delivery for those children who need it.
For its part, GSK has said that the eventual price of RTS,S will cover the cost of manufacturing together with a small return of five per cent that will be reinvested in research and development for next generation malaria vaccines or vaccines against other neglected tropical diseases.
Reaching this point has not been straightforward. We have only got this far thanks to our partners – the PATH Malaria Vaccine Initiative – and research centres across Africa that have helped enable the roll-out of late-stage trials. Looking ahead, more global and local partners will be needed to help realise broad and timely access to RTS,S across sub-Saharan Africa.
These partnerships could form a blueprint for future, similar projects. Whether or not the candidate vaccine is recommended, the fight against malaria will continue and the quest for new ammunition will go on. No single intervention is a silver bullet. More advanced vaccines, treatments and other methods will be needed if we are to finally suppress malaria.
Sophie Biernaux is head of the malaria vaccine franchise at GSK













